Oklahoma Center for Microbiome Research
Pilot Projects
Project 1: Phenotyping gut microbiome contributors to Fragile X Syndrome
Research Project Leader: McCullagh, Liz, PhD
Mentor: Dr. Noah Youssef
Disrupted gut microbiota are one of the most common symptoms across autism spectrum disorders (ASDs) yet, targets of gut imbalances have not led to fruitful treatment options for people with ASDs. Fragile X Syndrome (FXS) is the most common monogenic form of ASD, and similarly shares gut symptomology with other ASDs. In this proposal, we will isolate and screen the gut microbiota in both cecum and feces of a mouse model of FXS (Aim 1) while simultaneously isolating predominate microbial species and accessing their functional pathways (Aim 2) to provide targets for future studies on treatment based on microbial changes.
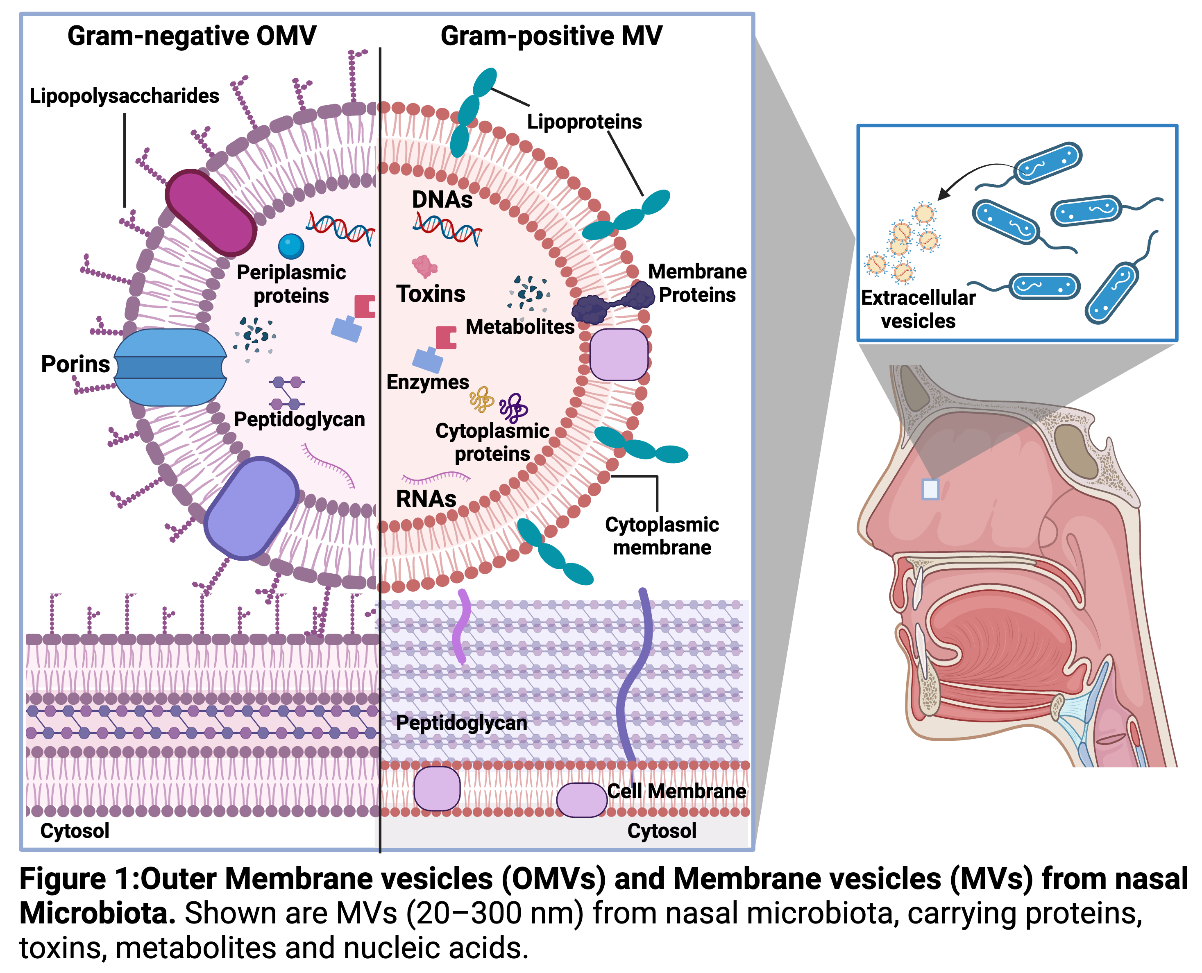
Project 2: Nasal microbiota-derived extracellular vesicles and their influence on epithelial function in chronic rhinosinusitis
Research Project Leader: Gujar, Vikram, PhD
Mentors: Dr. Lurdes Queimado
Dr. Vikram Gujar is a biomedical scientist with expertise in extracellular vesicles (EVs) and epithelial inflammation. His doctoral research focused on the molecular mechanisms underlying chronic inflammation and epithelial dysfunction. As a postdoctoral researcher at Northwestern University, Dr. Gujar studied EV-mediated signaling in airway inflammation, contributing to the growing understanding of how small extracellular vesicles influence immune responses. Currently, as an Assistant Professor at Oklahoma State University Center for Health Sciences, Dr. Gujar's research focuses on the role of bacterial-derived EVs in nasal inflammation and their impact on epithelial barrier integrity and immune modulation. Dr. Gujar's OCMR pilot project investigates EVs produced by Staphylococcus aureus and Cutibacterium acnes in the nasal microbiome and their potential role in chronic rhinosinusitis (CRS). While bacterial EVs have been studied in other systems, their impact on nasal epithelial cells and their contribution to chronic inflammation remain poorly understood. This project aims to characterize the molecular composition of bacterial EVs and evaluate their effects on nasal epithelial barrier function and cytokine release using air-liquid interface (ALI) cultures of human nasal epithelial cells. Findings from this study will provide crucial insights into bacterial EV-mediated inflammation and identify potential pathways for therapeutic intervention. The Anaerobic Core at OCMR is essential for this project, as it provides the infrastructure to culture anaerobic nasal bacterial strains under physiologically relevant conditions. Dr. Gujar's expertise in EV biology, combined with OCMR's specialized resources, will ensure the successful execution of this study and lay the groundwork for future NIH applications investigating the role of nasal microbiota-derived EVs in respiratory inflammation.
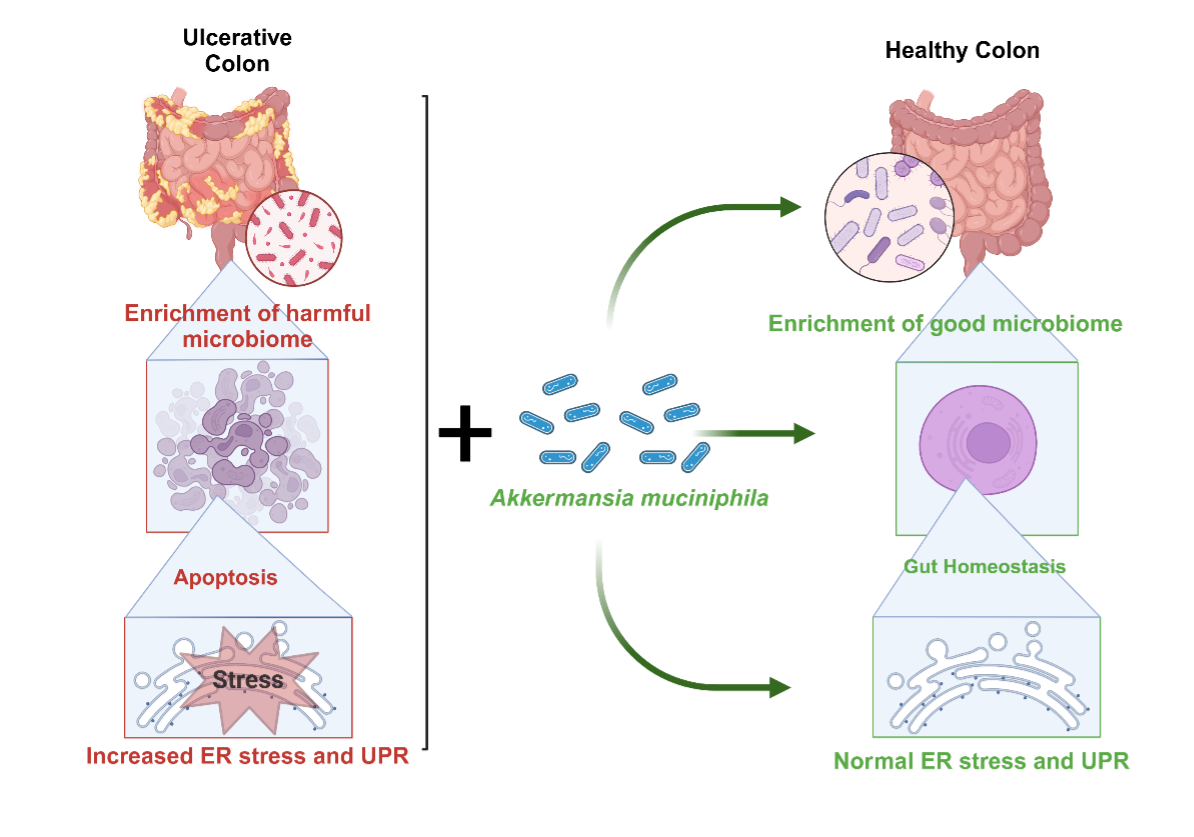
Project 3: Modulation of gut-brain axis by Akkermansia muciniphila through unfolded protein response
Research Project Leader: Das, Subhas, PhD
Mentors: Dr. Mostafa Elshahed
Dr. Subhas Das is an Assistant Professor of Biochemistry in the Department of Biochemistry and Microbiology at OSU-CHS. After completing his postdoctoral training in Dr. Sabita Roy’s lab at the University of Minnesota, Minneapolis, he joined OSU-CHS, shifting his research focus to epigenetic modulations in inflammation. His work primarily investigates the epigenetic signatures and landscapes of novel colon inflammation markers. In recent years, inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis, have become global health concerns, with a sharp rise in incidence, particularly among younger populations. The limitations of current treatment strategies underscore the urgent need for novel inflammatory markers that can serve as therapeutic targets. Using a TNBS-induced preclinical model of colitis, this study explores the effects of the probiotic Akkermansia muciniphila on dysregulated unfolded protein response and ryanodine receptor pathways, evaluating whether their inhibition can alleviate colitis pathobiology. Additionally, we will assess the influence of Akkermansia muciniphila on neurobehavioral outcomes, such as anxiety and social behaviors. By integrating microbiota-driven interventions with insights into cellular signaling pathways, this research aims to develop innovative therapeutic strategies targeting both gastrointestinal inflammation and neuropsychiatric symptoms, ultimately enhancing the quality of life for individuals with colitis and its comorbidities.