Ionizing Radiation
Ionization
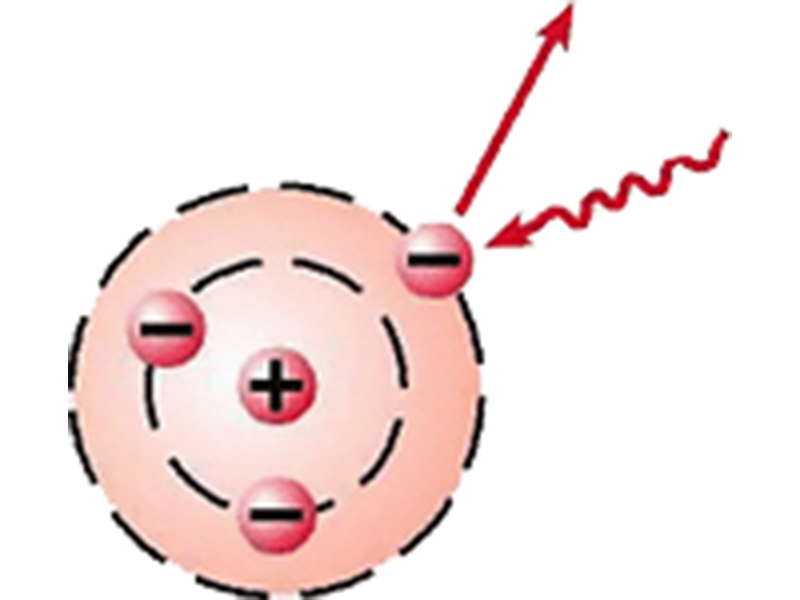
What does "ionization" mean? As shown in Figure 1, the atom can be visualized as a positively charged nucleus surrounded by one or more electrons that travel in circular orbits around the nucleus.
The nucleus is essentially a group of protons and neutrons (nucleons). While protons have a positive charge and electrons have a negative charge, neutrons, by contrast, have no net charge, though they do have a non-zero magnetic moment. This finding indicates that it, like the proton, is not an elementary particle, but instead has a substructure of other electrically charged particles called quarks. Stable atoms have equal numbers of protons and electrons, making the atom electrically neutral. If an atom should lose an electron, it would make the atom negatively deficient, with a net positive charge as the result. Losing another electron will produce a larger net positive charge to the atom. How can an electron be removed from its orbit? A quantum of energy is required, which is provided by an incident photon. A simple illustration of the ionization process is shown in Figure 2.
The converse is true for the addition of an electron. The addition of one or more electrons to a neutral atom will give the atom a net negative electric charge. An atom that has lost or gained one or more electrons is called an ion, and the process of gaining or losing electrons is called "ionization." Radiation that contains sufficient energy to remove an electron from its occupied energy level is called "ionizing radiation."
Radiation
"Radiation" can be a frightening word. The average person may immediately think of atomic bombs or nuclear power plant accidents. What the average person may not realize, however, is that radiation is also a natural phenomenon. Any object that is found to spontaneously emit energy is said to radiate that energy.
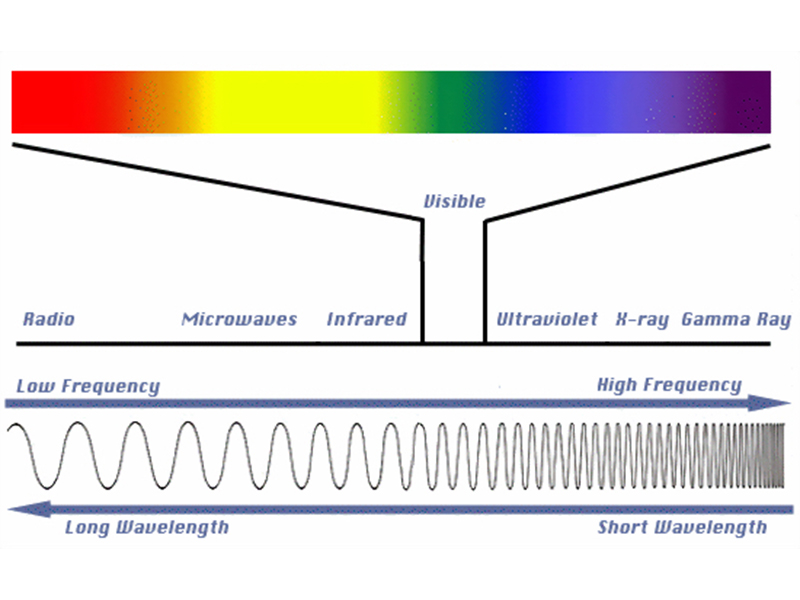
X-rays are a familiar form of radiation. X-rays have frequency (or energy) that allows them to pass through body tissues, but not through bones, which is why they are used in medicine to create images of, among other things, broken bones. X-rays are just one type of radiation found in the electromagnetic spectrum, which is illustrated in Figure 3.
Ionization takes place when an electron absorbs an amount of energy that exceeds the electronic binding energy of that orbital. Microwaves, which are commonly used to heat food, are another form of electromagnetic radiation, and so is visible light. Another kind of radiation comes from radioactive atoms. Certain elements, such as uranium or radium, have such a large nucleus that they are inherently unstable. Stability requires that a minimum energy state is attained. In order for a radioactive atom to reach this stable energy state, portions of the nucleus can be spontaneously ejected. This is called alpha particle radiation. An alpha particle is identical to the nucleus of a helium atom and consists of two protons and two neutrons. Radioactive atoms can also emit electrons—called beta rays in this context—or high energy electromagnetic radiation (gamma rays) in order to achieve a stable energy state.
Ionizing Radiation: Safety and Treatment
It may be surprising to learn that our world is inundated with ionizing radiation. It comes from our own Sun and from outer space in the form of galactic cosmic rays (GCRs). However, the Earth protects its occupants from most of these cosmic rays by using its atmosphere as a shield. Incoming cosmic rays react with the air we breathe. The resulting physical and chemical reactions change the cosmic rays into particles with reduced energy that do little or no damage to the population below.
Commercial aircraft routinely fly at altitudes up to 40,000 ft. This could be a problem since flight crews on those airplanes are subject to larger amounts of ionizing radiation at these altitudes than those of us on the ground. The occasional flight for the average person is nothing to be concerned about. Likewise, a routine x-ray at your dentist or physician is also perfectly safe. The long-term cumulative effect of radiation exposure to humans could be worrisome, however.
An incoming cosmic ray can wreak havoc on the human cell. Particularly at risk are cells that are in the process of dividing. This is why pregnant women have an increased risk of biological damage due to ionizing radiation: the developing fetus inadvertently provides many cellular targets.
Ironically, the damage that ionizing radiation can do to rapidly dividing cells also makes it a valuable treatment avenue for some cancers. Cancerous tumors are groups of cells that grow pathologically, making some forms of ionizing radiation such as protons useful in cancer therapy.